It’s happened to at least one of our smartphones or tablets. Our pride and joy piece of tech gets dented with an ugly crack or scratch. Smartphones are so essential to modern life that we stuff them into bulging pockets and handbags, not noticing what else we also can’t live without. Bunches of keys and coins don’t rub along too well with shiny glass surfaces. Happily advances in toughened glass chemistry are making these accidents less common.

A group from the Department of Applied Physics at Tohoku University in Japan recently reported a new way to study what makes the glass in our smartphones increasingly withstand everything our packed lives and handbags can throw at them. Writing in Nature Communications Physics, Professor Nobuaki Terakado described the valuable additional information that Raman microscopy gives glass technologists like Corning Inc. compared to existing materials characterisation methods.
Corning make the famous toughened Gorilla Glass that is found in an incredible 2 billion portable electronic devices. Gorilla Glass is much tougher than ordinary glass thanks to a clever surface modification that puts a compressive stress into a thin layer less than 50 microns thick. There is a cool video on the Corning website showing how the process works. Normal glass (like a wine glass or tumbler is made from) is a mixture of sand (sodium silicate) and boron trioxide. Corning glass used in smartphones contains aluminium oxide, making a harder and more resistant alumino-silicate glass. The really clever bit though is the surface modification step. At high temperature, the Corning glass surface is exposed to potassium ions in potassium oxide that swap places with sodium ions in the surface of the glass. When the glass cools down, the Gorilla Glass is left with a thin surface layer that has a residual compressive stress because the potassium ions are slightly bigger than the sodium ions in the rest of the material.
It is this stress that toughens the surface and makes it resist scratching and cracking. So stress is good, who knew?
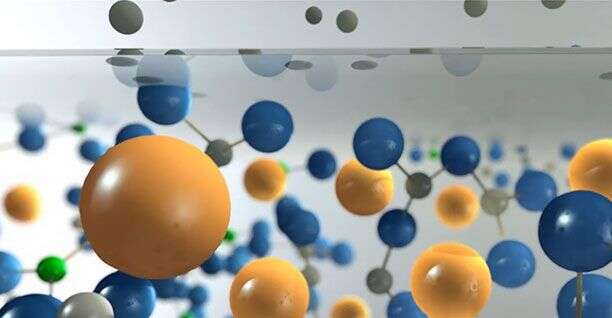
Understanding what happens when the alumino-silicate molecules and the alkali (sodium Na/ potassium K) ions get swashed together is crucial to predicting the enhanced properties of Gorilla Glass. Terakado and his team have shown that Raman microscopy reveals changes in the bonding of the silicate molecules and the ions. Not only can you see the effect of the residual stress you can non-destructively see the changes in the chemical bonds in the material as a function of depth. They managed this by measuring changes in the Raman vibrational fingerprint in the surface layer compared to the bulk. Other materials characterisation techniques can measure the amounts of potassium in the glass but only vibrational Raman microscopy can reveal the way the molecules and ions are crucially bonded together to create incredible strength and resistance.
The recent Nature Communications Physics paper is available for download. Follow the link to find further reading on Corning Gorilla Glass 3. Cracked smartphone image : ( used with permission from https://commons.wikimedia.org/wiki/File:Samsung_Galaxy_S2_shattered_screen.jpg .