Mineral deficiency in bean leaves classified by multispectral imaging

Plants tell us when they are lacking vital nutrients but we can’t always hear what they are saying. Nitrogen, phosphorous and potassium (N, P, K) are well know macronutrients and the appearance of plants lacking any one of them is also well known. Plants lacking nitrogen have small leaves and stunted growth, those lacking phosphorous have poorly developed root systems and plants deficient in potassium fail to flower well.
Micronutrients, including magnesium, boron and iron (Mg, B and Fe) also affect the way plants grow and function but act together with N P and K in ways that can be complicated. This means that chemical analysis is required to identify which element is lacking and how much should be added to a growing crop or to a field before sowing seed. Quantifying the elements present in plant material is straightforward to do in the lab but time consuming. If analysis is prompted by how the plant looks to the eye, it is also too late to correct a nutrient problem. To be useful, micronutrient analysis must be carried out at an early stage in plant development.
Researchers from the Department of Plant Development at the University of Zagreb recently reported that multispectral imaging of plant leaves can be a quick, early and non-destructive way to classify nutrient deficiency in young bean plants. Writing in the latest edition of the journal Frontiers in Plant Science, Boris Lazarevic and team described how multispectral imaging of french bean leaves can be used to distiguish normal healthy plants from those lacking nitrogen, phosphorous, potassium, magnesium or iron. Just three days after introducing nutrient deficient conditions, multispectral imaging correctly classified 92% of bean plants suffering from deficiency. After twelve days, 100% of bean plants could be correctly classified as healthy or deficient in N P K Mg and Fe.
How did they achieve this?

The team from Zagreb used an instrument similar to the PlantExplorer (shown above) to image juvenile leaves of french bean plants in containerised trays. Each tray contained plants growing in hydroponic media and was imaged at 3, 6, 9 and 12 days after the introduction of test solutions. A control solution with a cocktail of standard macro and micronutrients was the basis for the other nutrient deficient test solutions. Individual trays were grown with solutions lacking N, P, K, Mg and Fe components.
A phenotype or set of physical characteristics was used to identify potential changes in the leaves resulting from growing in solutions deficient in each mineral. These spectral parameters were either the reflectance of the leaves at different wavelengths (640, 550, 475, 510-590, 730, 769, 710 nm) or parameters (eg. green leaf index GLI, chlorophyll index CHI, anthocyanin index ARI, hue, saturation and intensity) derived from the images.
So far so good but how to extract useful information from the image data and how to evaluate the information? Lazarevic and team chose a statistical method known as linear discriminant analysis (LDA). LDA is a powerful way to use parameters or combinations of parameters that group together data from one set of plants and distinguish that set of plants from other sets of plants. In the case of the mineral deficiency study, the sets represented plants in each tray.
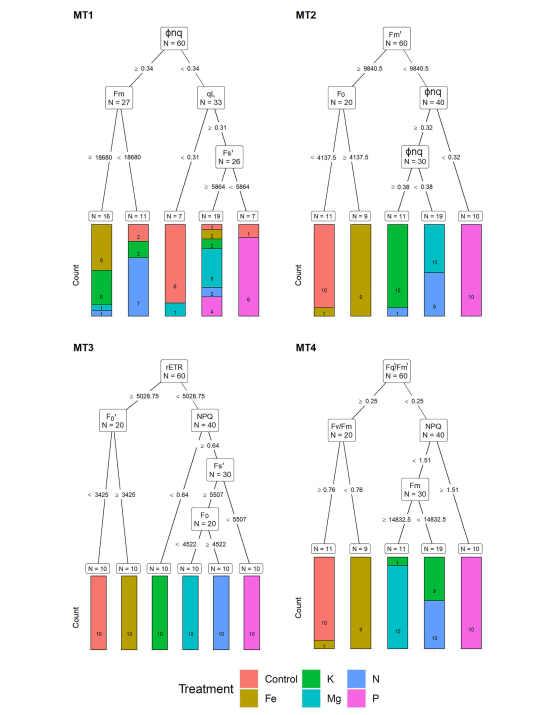
The figure above shows how multispectral parameters were used to classify plant leaf images. Each three day timepoint is denoted by MT1, MT2, MT3, MT4. It is interesting to note that different discrimination criteria were used for different measurement dates. Different colours represent the different missing minerals. After 12 days, using LDA, it was possible to correctly classify virtually all the plant images into control, N P K Mg and Fe deficient groups. After just 3 days (MT1) most of the plant images were correctly classified but not with the same criteria as those used on other dates.
In addition to multispectral measurements on the plant trays, the Zagreb group also evaluated chlorophyll fluorescence and morphological measurements as potential techniques for mineral deficiency classification. Chlorophyll fluorescence is of interest because it can reveal levels of plant activitity or function. Morphological measurements, such as plant height, have long been used by farmers to check the progress of crops.
However neither method was as successful as multispectral imaging in classifying mineral deficiency. The paper from the Department of Plant Development at the University of Zagreb reveals that multispectral imaging can be used to classify different mineral deficiencies in plants. Consequences of mineral deficiency can be detected after only three days but the fact that each measurement date requires a different set of classification criteria suggests that the methods tested are not yet robust enough to use as generic measures of mineral deficiency.
It will be fascinating to see how far the multispectral imaging methods can be developed into routine diagnostic techniques for farmers.
The full Journal of Plant Science article can be found here.
Information about the BACO multispectral imaging instrument available from Corbeau Innovation can be found here.