Cutting edge: Raman directed cancer surgery
Successful cancer surgery depends crucially on accurate removal of all the cancerous material. This can be tricky in cases where the tumor has invaded healthy tissue with its crabby legs and pincers. Simply erring on the safe side and removing large amounts of surrounding tissue isn’t always a good solution because this can affect the functioning of adjacent tissue.
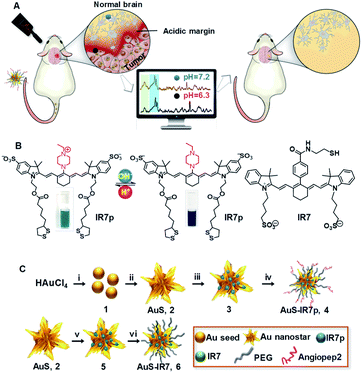
Avoiding unnecessary damage to healthy tissue surrounding a tumor in the brain is particularly important. It is also particularly difficult because the human brain has upwards of 85 billion nerve cells and something like a million billion interconnections. There are however some tell-tale signs to help guide surgery. It has been known for some years that tumor cells are frequently more acidic than normal healthy cells and this month a group from the Fudan University in Shanghai reported a novel way to guide the removal of brain tumor cells based on the fact they are more acidic than normal cells.
Writing in the journal Chemical Science Ying Mao, Xiao Yong Zhang and Cong Li describe how they designed a molecular probe that selectively reveals differences in acidity and used it to guide the removal of rat brain tumors. Importantly, surgery guided by the use of the molecular probe during the operation gave more complete removal of the tumor compared to standard pre-operative MRI imaging and with less removal of healthy tissue compared to use of a control dye probe during surgery.
They synthesised special dye molecules and attached them to gold nanoparticles, which made them easy to detect using a technique known as surface-enhanced resonance Raman scattering (SERRS). Normally Raman scattering gives scientists a very weak signal but when attached to the gold particles the light emitted by the probe molecules increased millions of times.
The Fudan group call their new cancer probe AuS-IR7p. Au refers to the coupling to gold nanoparticles; IR7 refers to the fact the dye is excited with near infrared light (which penetrates tissue much better than shorter wavelengths); and the p stands for the fact that when the dye is in an acidic environment (protonated) it apparently changes its orientation on the gold nanoparticle which changes the relative intensity of two of the characteristic peaks in the Raman spectrum. This proved very useful because taking the ratio of the two peaks gave a measure of the acidity of the brain tissue that was independent of dye concentration and overall fluorescence intensity.
The neurosurgery project at Fudan University used a classic approach to innovation. While the acidic nature of cancer cells was well-known, it was only last year that the Koch Institute at MIT reported the detection of tumor cell boundaries using fluorescent dye. Putting together new information from an established area of need and novel solutions to problems using existing dye probes they have developed a potentially valuable innovation.
It remains to be seen if further studies confirm the advantages of Raman directed brain surgery but as a case study in innovation it makes fascinating reading.
The original Chemical Science journal article published by the Royal Society of Chemistry can be found here. Additional experimental details can be found here. Background on the MIT group research can be found here and the literature paper is published in Cancer Research.